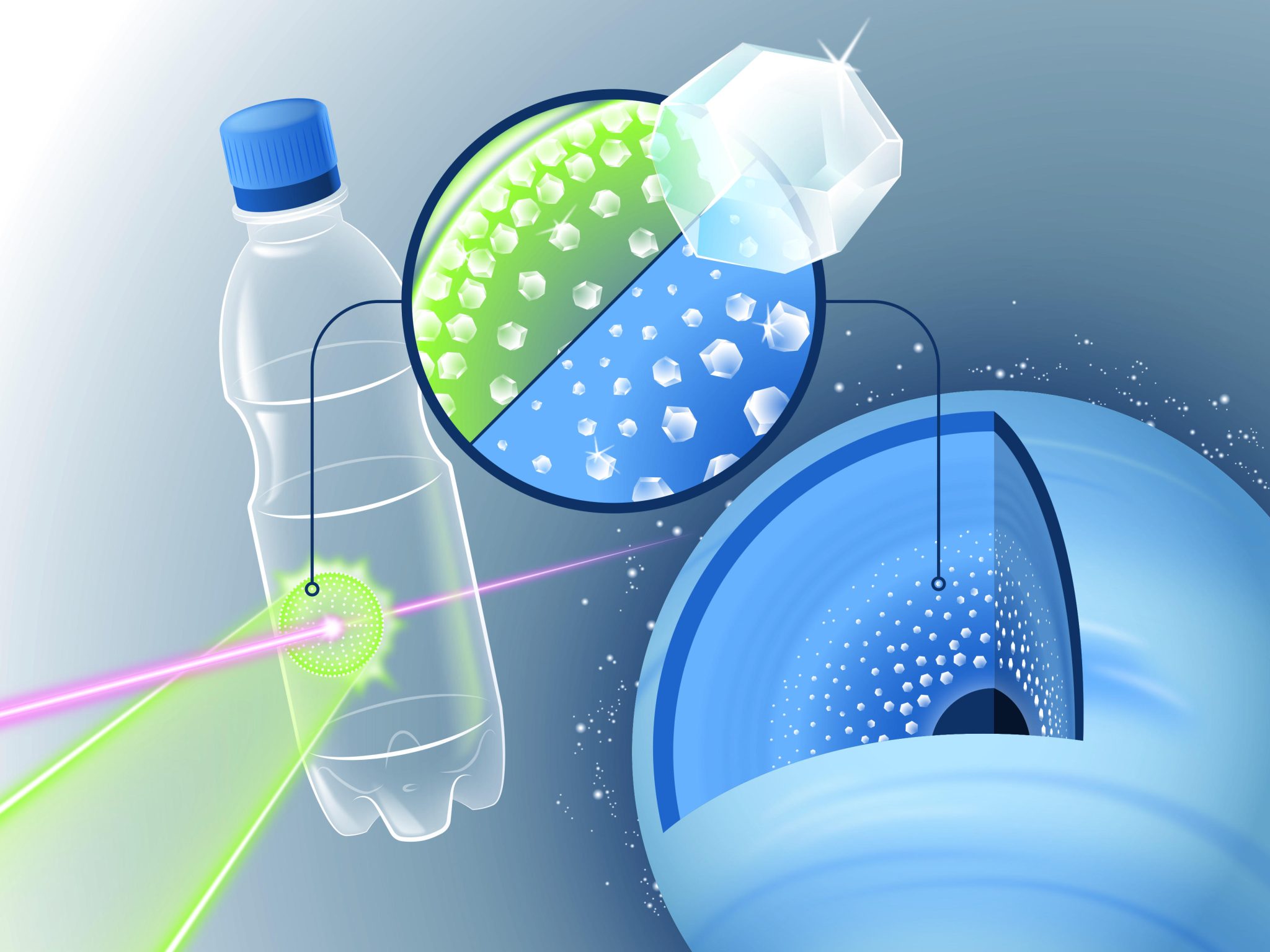
Dans l’expérience, une fine feuille de plastique PET simple a été cuite à l’aide d’un laser. De puissants éclairs laser qui frappent un échantillon d’un matériau semblable à une feuille le chauffent brièvement jusqu’à 6000 degrés Celsius, générant ainsi une onde de choc qui comprime le matériau à des millions de pression atmosphérique pendant quelques nanosecondes. Les scientifiques ont pu déterminer que les minuscules diamants, les soi-disant nanodiamants, se sont formés sous une pression extrême. Crédit : HZDR/Blaurock
L’équipe de recherche utilise des flashs laser pour reproduire l’intérieur des planètes glacées, inspirant une nouvelle méthode de production de minuscules diamants.
Que se passe-t-il à l’intérieur des planètes comme[{ » attribute= » »>Uranus and Neptune? An innovative experiment was carried out to find out by a global team led by the Helmholtz-Zentrum Dresden-Rossendorf (HZDR), the University of Rostock, and France’s École Polytechnique. They used intense laser flashes to study what occurred when they shot a laser at a thin sheet of simple PET plastic.
As a consequence, the scientists were able to support their prior hypothesis that diamonds really do rain within the ice giants at the edge of our solar system. Another was that this technique would provide a brand-new approach to making nanodiamonds, which are needed, for example, in very sensitive quantum sensors. The team’s findings were recently published in Science Advances.
Extreme conditions occur in the interior of large icy planets like Neptune and Uranus, with pressure millions of times higher than on Earth and temperatures that can reach several thousand degrees Celsius. However, states like these can be briefly reproduced in the lab by using intense laser flashes to hit a sample of a film-like material, heat it to 6,000 degrees Celsius in the blink of an eye, and create a shock wave that compresses the material to a million times the atmospheric pressure for a few nanoseconds.
“Up to now, we used hydrocarbon films for these kinds of experiments,” explains Dominik Kraus, a physicist at HZDR and professor at the University of Rostock. “And we discovered that this extreme pressure produced tiny diamonds, known as nanodiamonds.”
However, since ice giants also contain significant quantities of oxygen, in addition to carbon and hydrogen, it was only partially able to replicate the interior of planets using these films. When looking for suitable film material, the researchers stumbled upon an everyday substance: PET, the resin used to make ordinary plastic bottles.
“PET has a good balance between carbon, hydrogen, and oxygen to simulate the activity in ice planets,” Kraus explains.
The team carried out their research using the Linac Coherent Light Source (LCLS), a powerful, accelerator-based X-ray laser, at the SLAC National Accelerator Laboratory in California. They utilized it to analyze what transpires when powerful laser flashes hit a PET film while simultaneously using two measuring techniques: X-ray diffraction to detect if nanodiamonds were created and so-called small-angle scattering to see how fast and how big the diamonds grew.
Oxygen facilitates the process
“The effect of the oxygen was to accelerate the splitting of the carbon and hydrogen and thus encourage the formation of nanodiamonds,” says Dominik Kraus, reporting on the results. “It meant the carbon atoms could combine more easily and form diamonds.” This further supports the assumption that it literally rains diamonds inside the ice giants. The findings are probably not just relevant to Uranus and Neptune but to innumerable other planets in our galaxy as well. While such ice giants used to be thought of as rarities, it now seems clear that they are probably the most common form of planets outside the solar system.
The team also encountered hints of another kind: In combination with the diamonds, water should be produced – but in an unusual variant. “So-called superionic water may have formed,” Kraus opines. “The oxygen atoms form a crystal lattice in which the hydrogen nuclei move around freely.” Because the nuclei are electrically charged, superionic water can conduct electric current and thus help to create the ice giants’ magnetic field. In their experiments, however, the research group was not yet able to unequivocally prove the existence of superionic water in the mixture with diamonds. This is planned to happen in close collaboration with the University of Rostock at the European XFEL in Hamburg, the world’s most powerful X-ray laser. There, HZDR heads the international user consortium HIBEF which offers ideal conditions for experiments of this kind.
Precision plant for nanodiamonds
In addition to this rather fundamental knowledge, the new experiment also opens up perspectives for a technical application: the tailored production of nanometer-sized diamonds, which are already included in abrasives and polishing agents. In the future, they are supposed to be used as highly-sensitive quantum sensors, medical contrast agents and efficient reaction accelerators, for splitting
CO2 for example. “So far, diamonds of this kind have mainly been produced by detonating explosives,” Kraus explains. “With the help of laser flashes, they could be manufactured much more cleanly in the future.”
The scientists’ vision: A high-performance laser fires ten flashes per second at a PET film which is illuminated by the beam at intervals of a tenth of a second. The nanodiamonds thus created shoot out of the film and land in a collecting tank filled with water. There they are decelerated and can then be filtered and effectively harvested. The essential advantage of this method in contrast to production by explosives is that “the nanodiamonds could be custom cut with regard to size or even doping with other atoms,” Dominik Kraus emphasizes. “The X-ray laser means we have a lab tool that can precisely control the diamonds’ growth.”
Reference: “Diamond formation kinetics in shock-compressed C─H─O samples recorded by small-angle x-ray scattering and x-ray diffraction” by Zhiyu He, Melanie Rödel, Julian Lütgert, Armin Bergermann, Mandy Bethkenhagen, Deniza Chekrygina, Thomas E. Cowan, Adrien Descamps, Martin French, Eric Galtier, Arianna E. Gleason, Griffin D. Glenn, Siegfried H. Glenzer, Yuichi Inubushi, Nicholas J. Hartley, Jean-Alexis Hernandez, Benjamin Heuser, Oliver S. Humphries, Nobuki Kamimura, Kento Katagiri, Dimitri Khaghani, Hae Ja Lee, Emma E. McBride, Kohei Miyanishi, Bob Nagler, Benjamin Ofori-Okai, Norimasa Ozaki, Silvia Pandolfi, Chongbing Qu, Divyanshu Ranjan, Ronald Redmer, Christopher Schoenwaelder, Anja K. Schuster, Michael G. Stevenson, Keiichi Sueda, Tadashi Togashi, Tommaso Vinci, Katja Voigt, Jan Vorberger, Makina Yabashi, Toshinori Yabuuchi, Lisa M. V. Zinta, Alessandra Ravasio and Dominik Kraus, 2 September 2022, Science Advances.
DOI: 10.1126/sciadv.abo0617

« Drogué des réseaux sociaux. Explorateur d’une humilité exaspérante. Nerd du café. Amical résolveur de problèmes. Évangéliste culinaire. Étudiant. »
